- CAPA Post-root cause identification, the module supports the creation and tracking of corrective and preventive actions. Automated workflows ensure timely assignment, implementation, and verification of actions to address immediate issues and prevent recurrence.
- Documentation Management: Centralized storage of all deviation-related documentation ensures data integrity, transparency, and accessibility, fostering accountability and compliance.
- Collaboration and Communication: Facilitating effective stakeholder communication, our module sends notifications, task assignments, and updates, while also providing a platform for discussions and comments related to each deviation.
- Compliance Tracking: Aligned with industry regulations and internal policies, our module provides templates and workflows compliant with standards like Good Manufacturing Practices (GMP) and ISO, ensuring adherence to regulatory requirements.
- Reporting and Dashboard: Real-time visibility into ongoing investigations and CAPA activities is offered through deviation reports and dashboards. Analytics tools enable identification of trends and patterns, facilitating continuous improvement initiatives.
DEVIATION MANAGEMENT
Welcome to the Deviation Management module of Arizona TrackQS Quality Management System (QMS) software. This critical component is tailored to assist organizations, particularly those operating in highly regulated industries in effectively managing and resolving deviations from established processes and procedures. With our Deviation Management module, organizations can easily track and investigate deviations or non-conformances, helping to identify root causes and implement corrective actions promptly. This module ensures that deviations are addressed effectively to minimize risks and enhance product quality.
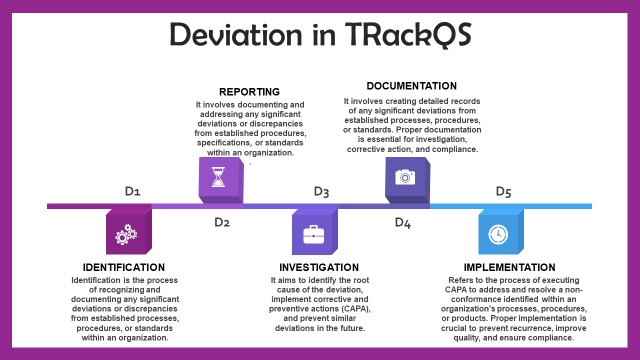
Deviations, unforeseen or unplanned events that can impact product quality, safety, or regulatory compliance, are meticulously addressed through the following features of our Deviation Management module:
- Incident Reporting: Users can promptly report and document deviations or incidents, ranging from process deviations to equipment malfunctions. Crucial details such as the nature of the deviation, location, date, and involved individuals are captured for comprehensive documentation.
- Investigation Workflow: Streamlining complex investigation processes, our module automates task assignment, notifications, and approvals to ensure a thorough investigation involving the right personnel, thus expediting resolution.
- Root Cause Analysis: Identifying the root cause is pivotal in preventing recurrence. Our module provides tools and templates for conducting root cause analysis, integrating methodologies such as 5 Whys, Fishbone Diagrams (Ishikawa), or Failure Mode and Effects Analysis (FMEA) for effective problem-solving.
- Risk Assessment: Assessing and quantifying risks associated with deviations aids in prioritizing corrective actions and determining urgency. Our module facilitates risk assessment, empowering organizations to make informed decisions swiftly.
Make an Appointment
Clients
Contact
Our Address
 A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
 Flat 1, 60 cardiff road, Newport
NP20 2 EG
Flat 1, 60 cardiff road, Newport
NP20 2 EG
Email Us
info@arizonaautomation.in
contact@arizonaautomation.in
Call Us
 +91 9909786331
+91 9909786331
 +971 509310159
+971 509310159
 +44 776 793 5850
+44 776 793 5850
 +1 732 642-7966
+1 732 642-7966
 +1 647 624-4789
+1 647 624-4789














