- Notification and Approval: Users can attach relevant documentation and supporting information to change requests, including impact assessments, risk analyses, and justifications, facilitating informed decision-making.
- Change Implementation: Manage the implementation process, updating documents, revising processes, or making system changes as necessary.
- Verification and Validation: Track verification and validation processes to ensure changes are implemented correctly and effectively, particularly in regulated industries.
- Compliance Tracking: Ensure adherence to industry regulations and internal policies, providing templates and workflows aligned with standards such as Good Manufacturing Practices (GMP) or ISO.
- Document Management: Centralize all change-related documentation within the QMS software, promoting data integrity and accessibility for compliance and auditing purposes.
- Reporting and Analytics: Gain real-time visibility into change requests, approvals, and implementations through reports and dashboards. Analytics tools identify trends and patterns, aiding decision-making and continuous improvement efforts.
- Audit Trail: Detailed audit trails capture all actions and changes within the module, critical for demonstrating compliance during regulatory audits and inspections.
- Integration: Seamlessly integrate with other QMS modules such as Document Management, CAPA (Corrective and Preventive Actions), and Training Management to ensure interconnectedness and coherence in quality processes.
CHANGE CONTROL
Introducing Arizona TrackQS (QMS) Software's Change Control Module – a pivotal tool that empowers organizations to manage and control changes to their processes, products, documents, or systems in a structured and compliant manner. Our Change Management module is aligned with FDA, MHRA, and ICH Q10 guidelines, providing organizations with a robust system to manage change effectively. From initiation to implementation, our software ensures that changes are evaluated, approved, and implemented in a timely and compliant manner.
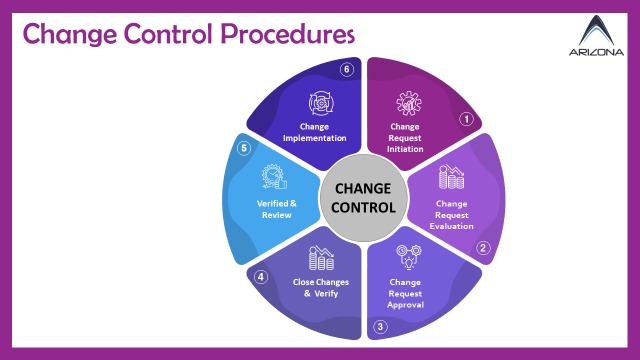
Here's a comprehensive breakdown of its functionalities:
- Change Control Initiation: Authorized users can initiate change requests encompassing various changes, including process modifications, product design alterations, document updates, and system changes.
- Workflow Automation: The module automates workflows for review, approval, and execution, ensuring that change requests are routed to the appropriate individuals or departments based on predefined criteria and roles.
- Documentation: Users can attach relevant documentation and supporting information to change requests, including impact assessments, risk analyses, and justifications, facilitating informed decision-making.
- Impact Assessment: Impact Assessment: Evaluate the potential impact of proposed changes on processes, products, regulatory compliance, and other aspects of the business.
- Risk Assessment: Facilitate risk assessment by providing tools and methodologies to evaluate the likelihood and severity of potential issues associated with the change.
Make an Appointment
Clients
Contact
Our Address
 A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
 Flat 1, 60 cardiff road, Newport
NP20 2 EG
Flat 1, 60 cardiff road, Newport
NP20 2 EG
Email Us
info@arizonaautomation.in
contact@arizonaautomation.in
Call Us
 +91 9909786331
+91 9909786331
 +971 509310159
+971 509310159
 +44 776 793 5850
+44 776 793 5850
 +1 732 642-7966
+1 732 642-7966
 +1 647 624-4789
+1 647 624-4789














