- Preventive Action: Implement preventive measures to forestall the recurrence of incidents or issues. Automated workflows ensure efficient development and deployment of preventive actions.
- Document Management: Centralize all CAPA-related documentation, including incident reports, investigation reports, and action plans, within the QMS software. This ensures data integrity and accessibility for compliance and auditing purposes.
- Compliance Tracking: Ensure alignment with industry regulations and internal policies through templates and workflows compliant with standards such as Good Manufacturing Practices (GMP) or ISO.
- Collaboration and Communication: Facilitate effective stakeholder communication by sending notifications, task assignments, and updates. A platform for discussions and comments fosters collaboration throughout the CAPA process.
- Reporting and Analytics: Gain real-time visibility into ongoing investigations and corrective/preventive actions through CAPA reports and dashboards. Analytics tools identify trends and patterns in incident occurrences, driving continuous improvement efforts.
- Audit Trail: Detailed audit trails capture all actions and changes within the CAPA module, ensuring transparency and compliance during regulatory audits and inspections.
- Integration: Seamlessly integrate with other QMS modules such as SOPs Management, Training Management, and Deviation Management to ensure interconnectedness and coherence in quality processes.
CORRECTIVE & PREVENTIVE ACTION
Introducing Arizona TrackQS (QMS) Software's CAPA Module – an indispensable tool for organizations operating in regulated industries like pharmaceuticals and manufacturing. Designed to identify, address, and prevent issues or non-conformities in processes, products, or services, Our CAPA Management module provides a structured approach to investigating and addressing issues, ensuring that corrective actions are implemented effectively to prevent recurrence and drive continuous improvement.
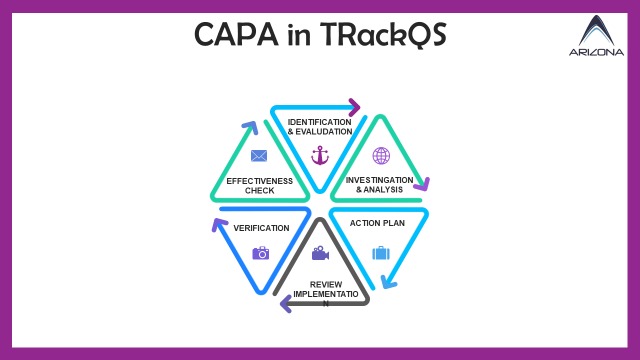
our CAPA module offers a comprehensive solution to enhance quality management practices. Here's an in-depth overview of its functionalities:
- Incident Reporting: Initiate the CAPA process by reporting incidents, issues, non-conformities, or deviations. Users can efficiently document problems ranging from product defects to regulatory compliance concerns or customer complaints.
- Root Cause Analysis: Facilitate thorough root cause analysis to identify underlying reasons behind incidents or issues. Integrated tools such as the "5 Whys," Ishikawa (Fishbone) diagrams, or Failure Mode and Effects Analysis (FMEA) assist in this process.
- Investigation Workflow: Streamline investigation workflows by assigning tasks, setting deadlines, and tracking progress. Automated notifications keep relevant parties informed and engaged throughout the process.
- Risk Assessment: Evaluate the potential impact and severity of incidents using risk assessment tools. This aids in prioritizing corrective actions and preventive measures effectively.
- Corrective Action: Take immediate corrective actions to address the identified issue or incident. The CAPA module facilitates the creation, assignment, implementation, and tracking of these actions.
Make an Appointment
Clients
Contact
Our Address
 A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
A-607, Siddhi Vinayak Business Tower
Ahmedabad, Gujarat 380051
India
 Flat 1, 60 cardiff road, Newport
NP20 2 EG
Flat 1, 60 cardiff road, Newport
NP20 2 EG
Email Us
info@arizonaautomation.in
contact@arizonaautomation.in
Call Us
 +91 9909786331
+91 9909786331
 +971 509310159
+971 509310159
 +44 776 793 5850
+44 776 793 5850
 +1 732 642-7966
+1 732 642-7966
 +1 647 624-4789
+1 647 624-4789














