
COMPUTERIZED SYSTEM VALIDATION
Computerized System Validation (CSV) ensures that computer systems used in regulated environments operate accurately, reliably, and in compliance with industry standards.
PLC/ HMI/ IPC/ SCADA/ DCA System Validation
HPLC, GC, FTIR, Spectrometer, EMPOWER, UV, LCMS-MS, Chromeleon, SAS, WinOnlin & more
SaaS, SAP, ERP, MES, LIMS, TMS, QMS, E-DMS
Environment Management System (EMS) Validation
Cloud, Server, Network Validation, Meta Data Qualification, Desktop Validation
Track & Trace, TracLINK, ReeTrak, L1 to L5 Integration Validation, Warehouse Management Software Validation
CSV Audit and CSV Training
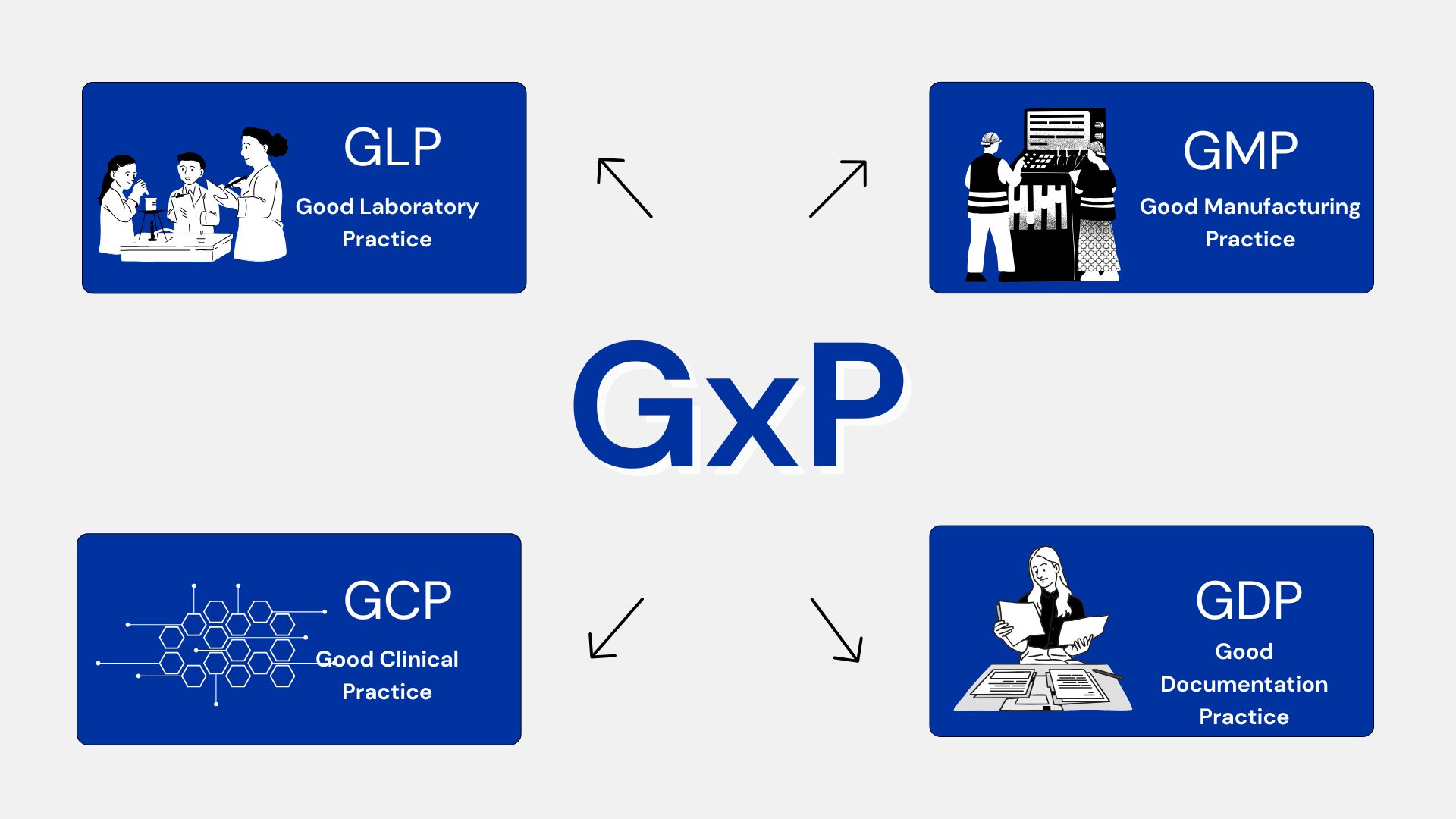
Identifying regulatory frameworks (NOM / FDA / WHO) before validating system requirements.
Aligning validation with Quality Management Systems (QMS) of each
organization.
Designed for use regardless of validation knowledge, covering: