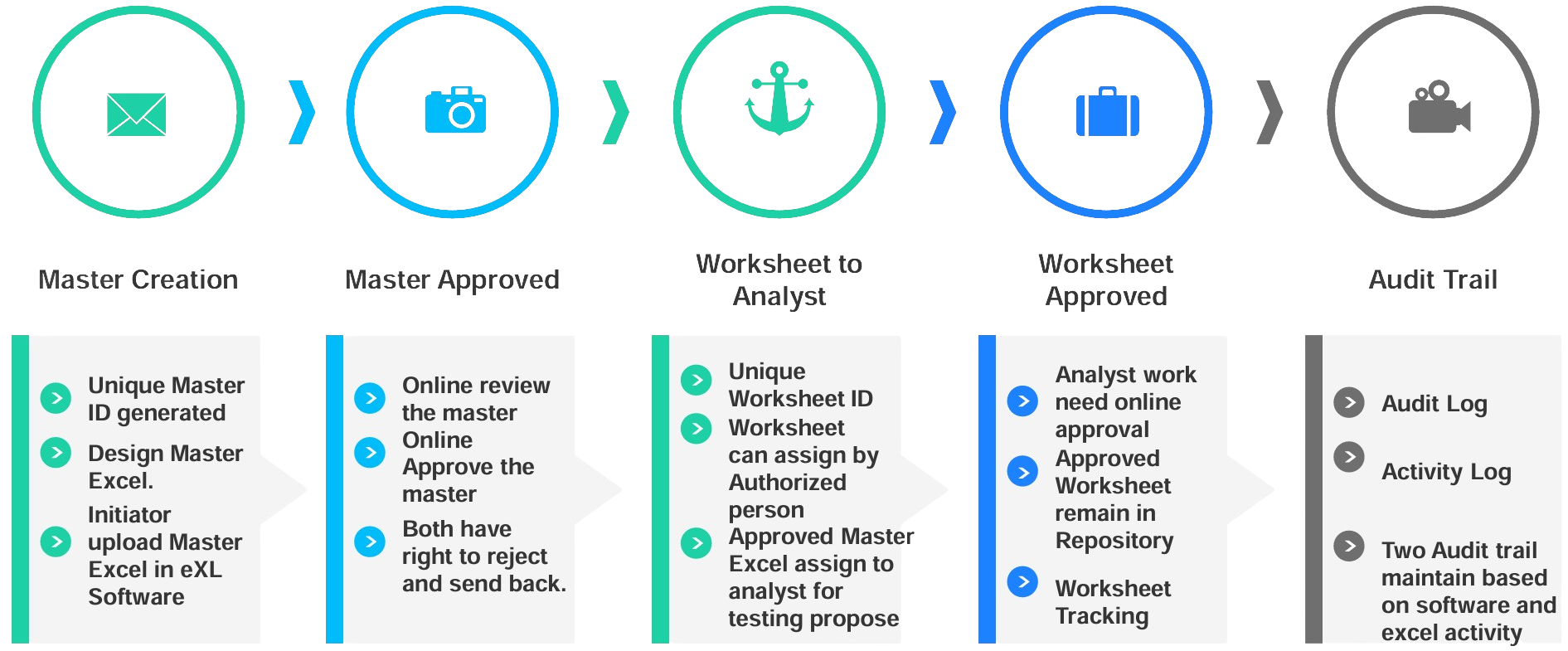
Arizona Automation & Technologies Spreadsheet Management Software (eXL) for Pharma industries to manage GxP data, used to perform GMP–related calculations, trend data, plan calibration and qualification work, and manage laboratory assets and compliance management. “eXL” comprehensive solution designed to address the specific needs of quality control (QC) laboratories. This spreadsheet software should eliminated possible risk from performing GxP work to increase business efficiency and reduce regulatory risk.